What is USP <797> and <800>?
USP <797> and <800> are General Chapters dealing with non hazardous and hazardous drugs in healthcare, enforceable by regulators and accreditation organizations. Chapters <795> and <797> have been in force for many years. Chapter <800> is being introduced to "help promote patient safety, worker safety, and environmental protection". This section defines processed intended to minimize the exposure to hazardous drugs in health care settings.
Some of the sections of USP <800> as well as <797> and <795> which have to do with environmental concerns are mentioned below. The information listed below is meant only as a general guidelines to the regulations. For more specific information the reader should refer to the regulations themselves. More information can be found here.
Any entity handling non-hazardous <795> or hazardous drugs <797> and <800> is currently required to comply with the requirements of USP <797> and <795>. In addition to these requirements USP <800>, is expected to go into effect December 1, 2019 and applies to handling hazardous drugs in healthcare settings. There are a few requirements that impact the physical areas where hazardous drugs are handled. Handled, in the case, means manipulated, packaged, mixed and contacted in any way, whether by hand or an implement.
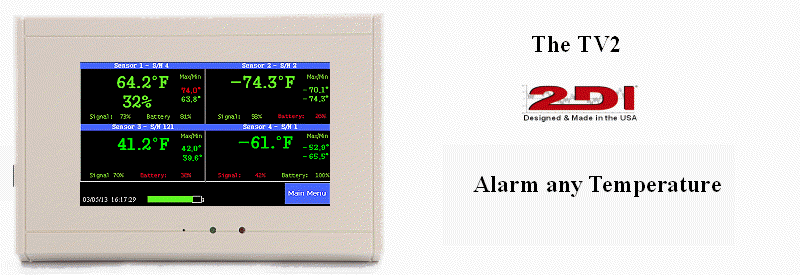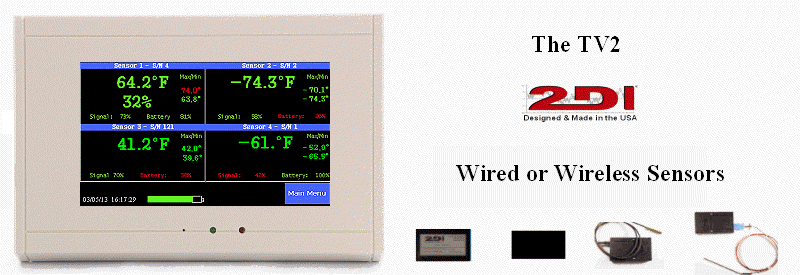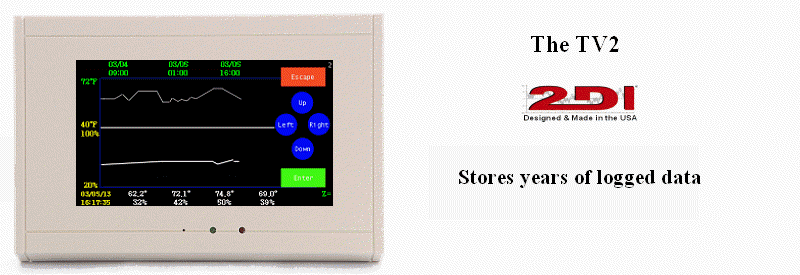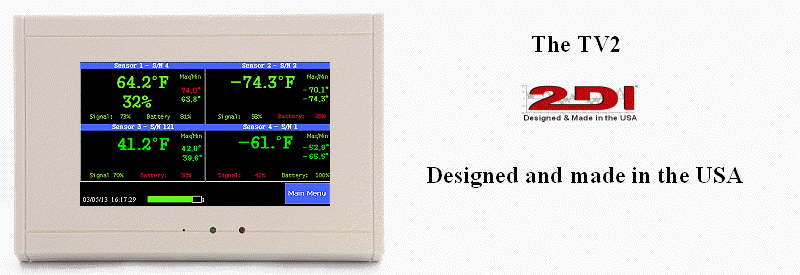
Monitoring Pressure
A new requirement of USP <800> has to do with Containment Secondary Engineering Control (C-SEC). In addition to this area having to be externally vented to the outside of the building, it must be maintained at a negative pressure between 0.01 and 0.03 inches of water column relative to all adjacent areas, at all times (24/7).
Currently, under USP <797>, a requirement exists for the presence of a pressure guage and at least daily monitoring of that guage. A pressure gauge must monitor the differential pressure between the ante-area and the cleanroom area and also between the buffer area and the ante-area of an IV room. So two different differential pressure gauges are required to be in compliance.
There is no requirement for a pressure gauge under USP <795>.
Temperature and humidity monitoring requirements/h3>
TTemperatures should be maintained below 20°C (68°F). Under chapter <797> there is not a requirement for humidity contol or monitoring, although it is generally accepted good practice to maintain Relative Humidity between 35% and 60%.
In addition to the USP regulations, the Joint Commission (JCAHO) requires a program for management of proper temperature and relative humidity levels in accordance with Facilities Guideline Institute Guidelines for Design and Construction of Hospitals and outpatient Facilites for all centralized sterile supply storage areas.
NNote: JCAHO is continually evaluating the effacy of its requirements and is expected to issue new requirements for humidity monitoring of all storage areas for sterile equipment in the near future.
Pharmacy Today article on Compounding nonsterile preparations: USP <795> and <800>